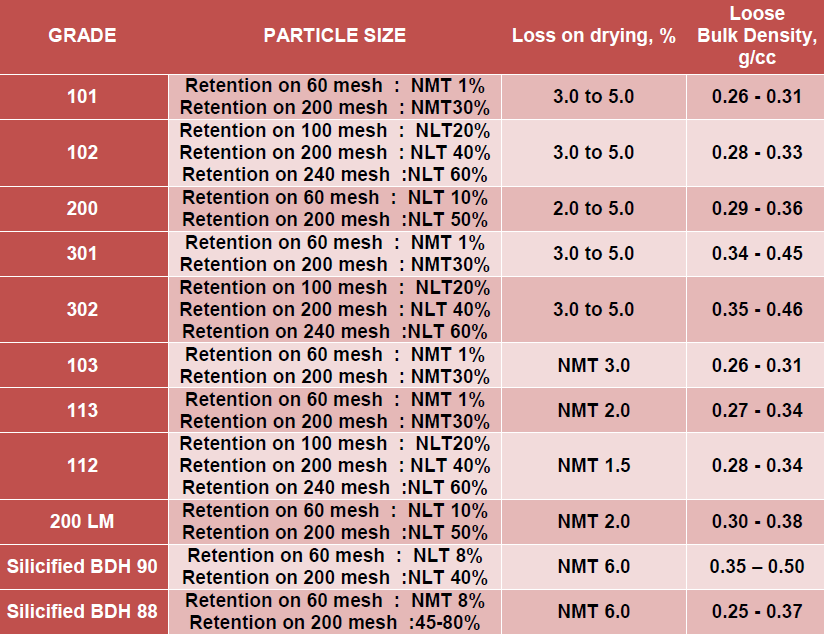
Microcrystalline Cellulose – Ambicel®
Micro Crystalline Cellulose, a versatile pharmaceutical excipient is manufactured under Brand Name “Ambicel”
- Ambicel PH for direct compression tableting
AMBICEL MCC’S Unique morphology renders tablets strength; low lubricant sensitivity and fast disintegration power. AMBICEL MCC makes tables strong by virtue of large number of binding sites and very high surface area.
AMBICEL MCC makes tablets less sensitive to lubricants due to its crystalline structure. AMBICEL MCC makes tablets porous and water permeates deep into tablet matrix quickly and fast disintegration of tablet is achieved
- Ambicel PH for wet granulation
AMBICEL MCC due to its wicking action thoroughly distributes granulating fluid throughout the powder and rapid and uniform wetting is achieved. AMBICEL MCC due to its very high surface area insures rapid and uniform drying of wet granulation. It prevents over wetting and maintains wet mass consistency due to its large surface area and absorptive capacity. Product also helps trouble free screening due to uniform wetting of powder. It distributes drug evenly throughout the particles of granulation and prevents drug or dye migration during drying. AMBICEL MCC possesses wet binding power and reduces the requirement of traditional wet binders.
-
Recommendation of ambicel in wet mix
TYPICAL WET BASE A mixture of 70% lactose with 30% AMBICEL is recommended in wet mix in order to get harder granules and fewer fines. ADDING OF AMBICEL Usually one-half of AMBICEL is added to wet mass and one-half as a post granulation addition where itprovides the typical direct compression benefits.
-
Other applications of ambicel
1] Low calorie food like cookies; makroni and spaghetti
2] Butter, Cheese, Margarine, Free flowing granular powders of Syrups, Honey, Molasses.
3] Salad dressing and chocolate drinks.
4] Thin layer Chromatography AMBICEL M C C products are generally recognised as safe (GRAS) by qualified experts and are in accordance with food and drug regulations.